Abstract
Introduction
Health insurance, or lack thereof, is a significant barrier to health care access in the United States. Patients without insurance or with inadequate coverage are more likely to delay or forego treatment, even with acute illness or significant symptoms, leading to worse health outcomes. We aimed to analyze if insurance types impacted one-month mortality and overall survival (OS) in younger patients with APL.
Methods
We utilized National Cancer Database to identify patients <65 years who were diagnosed with APL between 2004-2015. We used multiple logistic regression analysis to evaluate the effects of insurance type on the probability of one-month mortality. OS was estimated by the Kaplan-Meier method. A full Cox regression model was used to determine the effects of insurance types on mortality.
Results
A total of 5380 patients were included. Median age was 44 years (0-64), 50% were female, 93% had Charlson-Deyo comorbidity index (CCI) of 0 or 1, and 58% were treated at academic centers. Insurance types included private (67%), Medicaid or other government insurance (19%), Medicare (7%) or uninsured (6%). Patients with Medicare were older and had increased comorbidities. Lower percent of patients with Medicare (23%) or Medicaid/Other government insurance (21%), compared to those with private insurance (56%) or uninsured patients (63%) were treated at academic centers.
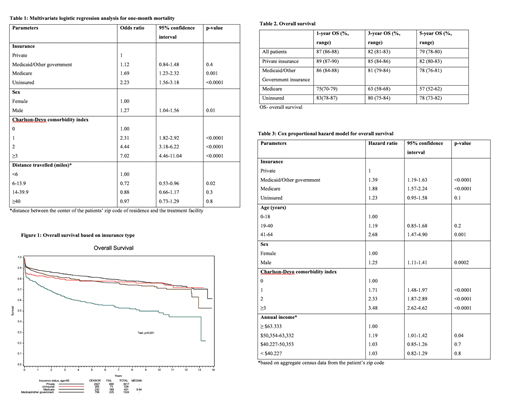
One-month mortality was higher for patients with Medicare (16%) or uninsured patients (14%), compared to those with Medicaid/Other government (8%) or private insurance (7%).Patients with Medicare (Odds ratio [OR] 1.69, 95% confidence interval [CI] 1.23-2.32, p=0.001) or uninsured patients (OR 2.23, 95% CI 1.56-3.18, p<0.0001) had worse one-month mortality compared to those with private insurance (Table 1). One-month mortality worsened with increasing comorbidities (OR 2.31 for CCI 1, OR 4.44 for CCI 2, and OR 7.02 for CCI ≥3 compared to patients with CCI of 0, p<0.0001). Female patients and patients traveling <6 miles to the treatment center had lower one-month mortality.
Median follow-up for surviving patients was 5.4 years (0.008-13.9). Three-year OS was 89% for private insurance, 81% for Medicaid/Other government insurance, 63% for Medicare, and 80% for uninsured patients (Table 2, Figure 1). Patients with Medicaid/Other government insurance (hazard ratio [HR] 1.39, 95% CI 1.19-1.63 p<0.0001), and Medicare (HR 1.88, 95% CI 1.57-2.24, p<0.0001) were associated with worse OS compared to patients with private insurance (Table 3). Compared to patients ≤18 years of age, the likelihood of death was worse for patients 41-64 years (HR 0.68, 95% CI 1.47-4.90, p=0.001). OS worsened with increasing comorbidities (HR 1.71 for CCI 1, HR 2.33 for CCI 2, and HR 3.48 for CCI ≥3 compared to patients with CCI of 1, p<0.0001). Male gender (p=0.0002) was associated with decreased OS.
Conclusion
In one of the largest database analyses, we identified insurance status as a significant factor affecting one-month mortality and OS in APL. Our results revealed a higher one-month mortality but similar longer-term OS in uninsured patients compared to patients with private insurance, which may reflect poor access to healthcare necessary for prompt diagnosis and timely initial treatment; similar longer-term OS may reflect a higher proportion of younger patients with less comorbidities in uninsured group. Patients with Medicaid/Other government or Medicare insurance had worse OS compared to private insurance. The reasons for worse OS may be multifactorial including problems with access to quality leukemia care or drug coverage, or the effects of other differences in the patient population including income and in case of Medicare patients, older age and comorbidity burden. Our results raise concern for healthcare disparities based on insurance types and highlight challenges associated with improving OS in patients with Medicaid, Medicare, or no insurance, which comprise a significant proportion of patients with APL.
Gundabolu: Samus Therapeutics: Research Funding; Pfizer: Research Funding; BioMarin Pharmaceuticals: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Company: Consultancy. Bhatt: Genentech: Consultancy; Abbvie: Consultancy, Research Funding; National Marrow Donor Program: Research Funding; Tolero Pharmaceuticals, Inc: Research Funding; Pfizer: Research Funding; Incyte: Consultancy, Research Funding; Jazz: Research Funding; Abbvie: Consultancy, Research Funding; Partnership for health analytic research, LLC: Consultancy; Servier Pharmaceuticals LLC: Consultancy; Rigel: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal